GRACE for AUD: proposing GLP-1-based alcohol treatment initiation in the ED
Immediate-access craving relief therapy can provide short-term risk reduction and a bridge to treatment.
We previously proposed the GRACE model for OUD—GLP-1 Rapid Access CAre—as a bridge between harm reduction and treatment for individuals with opioid use disorder (OUD). GRACE for OUD would provide immediate access to GLP-1RAs like Ozempic to reduce opioid cravings. Unlike existing medications for OUD, it can offer easy initiation in mobile and street-medicine models for people with severe addiction and housing instability.
GRACE for alcohol use disorder (AUD) is similar—a strategy offer long-term reductions in alcohol craving during a medical encounter for acute need. This can dramatically increase patient willingness to embrace treatment and makes meaningful treatment initiation possible while a patient is recovering from severe alcohol intoxication.
The top 10% of drinkers consume more than 55% of total alcoholic drinks. Offering GRACE to patients in emergency departments (ED) with alcohol-related complications would reach the population at highest risk of acute and chronic harm from alcohol use at a moment when they may be most likely to accept treatment. There is potential for disproportionately positive public health impact.
GLP-1RAs reduce alcohol consumption and AUD incidence
GLP-1 receptor agonists (GLP-1RAs), such as Ozempic and Mounjaro, have shown dramatic reductions in alcohol cravings, use, and AUD rates in randomized controlled trials, real-world retrospective patient health record studies, and animal models. The efficacy of these drugs appears to exceed that of existing medications for AUD, and they are already being prescribed by many doctors for this purpose.
GLP-1RAs show large reductions in alcohol craving and consumption, with effect sizes that appear to be 3-5X stronger than naltrexone, the current standard of care for AUD. Naltrexone is a life-saving medicine for many of the people who take it, but adoption by patients is very limited, in part because it requires 3-7 days of sobriety before it can be initiated.
Physicians, including ones at leading substance use disorder treatment clinics such as the Caron Treatment Centers and Stanford Addiction Medicine, are prescribing GLP-1s including Ozempic and Mounjaro for AUD and other substance use disorders. Because they do not yet have an FDA indication, however, these prescriptions are off-label, with patients paying our of pocket, or are written for comorbidities of obesity or diabetes.
GLP-1s Can Increase the Number of Patients Accepting AUD Treatment
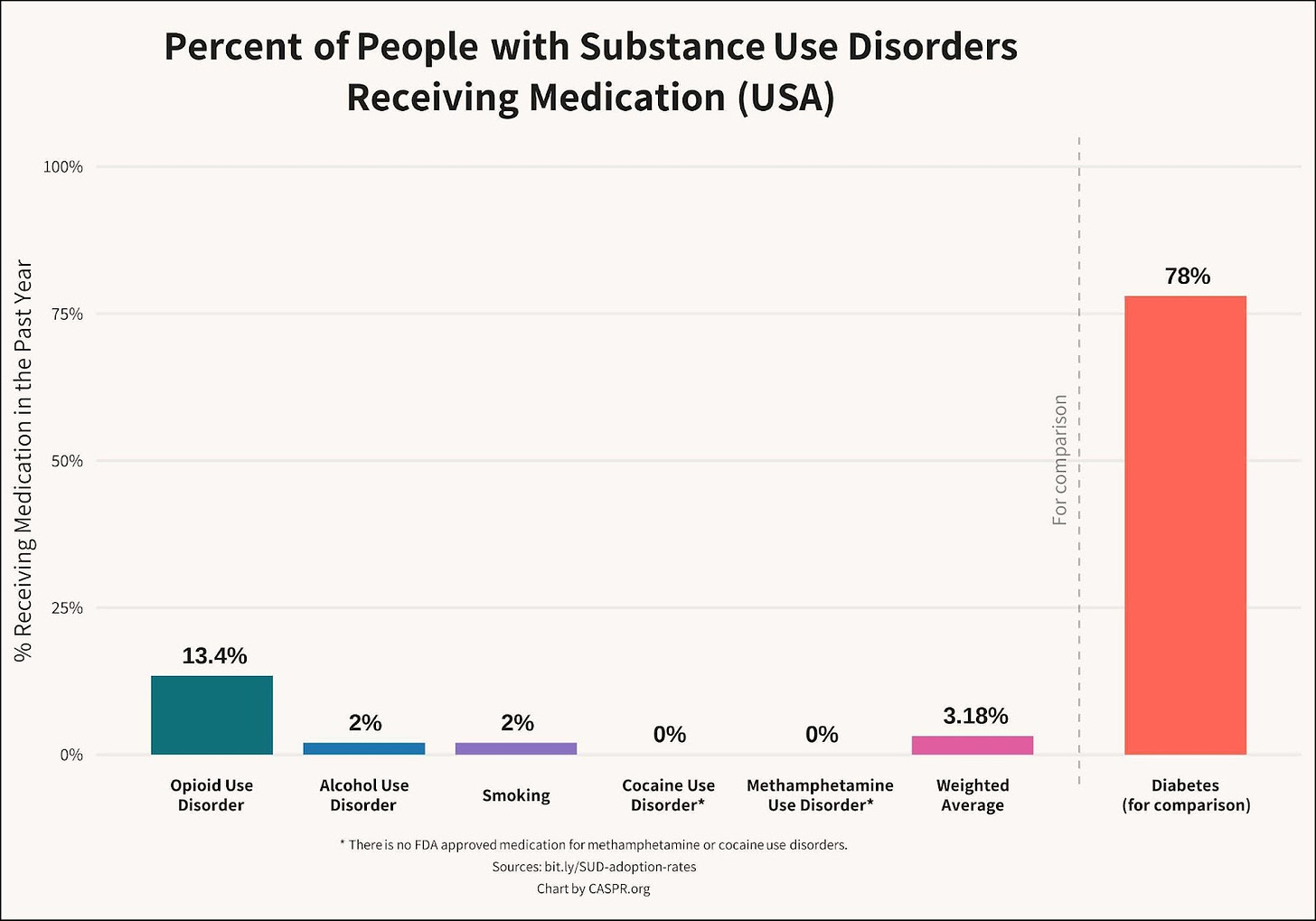
GLP-1s offer the potential to dramatically increase the number of people who are willing to take, and continue to take, medications to reduce alcohol use. Currently, only 2% of people with AUD take medication because existing options are unappealing to patients and have modest efficacy. GLP-1RAs offer numerous appealing advantages, including ease of initiation, ease of discontinuation, reductions in excess weight, mitigation of suicidality, and improvements to mental health.
We encourage you to read GLP-1RAs for Addiction: the Medical Evidence for Opioid, Nicotine, and Alcohol Use Disorder, which is the most comprehensive review available of the scientific and strategic advantages of these medications. It also provides academic references for the evidence discussed above.
GRACE: GLP-1 Rapid Access Care
The GRACE model, proposed here, would offer point-of-contact initiation of GLP-1RAs for people who present in the ED with alcohol poisoning or signs of AUD.
GRACE consists of the following:
Patients presenting in the ED with acute alcohol harm or indications of AUD would be offered GRACE.
Patients who accept GRACE would be given an injection of semaglutide or tirzepatide during their initial encounter. They would also be given the 3 additional once-per-week injection pens from their initial month-long prescription to take home.
Patients would be set up to receive ongoing monthly refills at their pharmacy and a phone number for ongoing support by text or voice.
Patients would be supported in scheduling a follow-up for ongoing care with a physician or clinic, if they are willing, but should be given ongoing GLP-1 access regardless of in-person appointment attendance. (GLP-1s have been shown to greatly reduce heavy drinking days even in non-treatment seeking individuals.)
For patients who are reachable by phone, providers would ideally conduct follow-up communication via telephone and text messaging to monitor treatment adherence, provide refills, and encourage patients to attend subsequent appointments. Patients would be encouraged to setup follow up appointments for additional SUD treatment and assessment but would not be required to make monthly in-person visits to receive GLP-1RA refills unless providers had specific concerns. In this population, ease of use and access is paramount both for individual patient continuation and for increasing the appeal of the program for potential new participants.
Potential Medical and Strategic Benefits of GRACE
GRACE offers a number of potential benefits to patients, providers, and harm reduction programs:
GRACE would provide both a rapid reduction in alcohol cravings that many people experience upon GLP-1RA initiation and and the potential to dramatically reduce the negative effects of excess alcohol use over the long-term.
GRACE can engage individuals who have the highest likelihood of imminent adverse outcomes. Immediate initiation during an ED visit or at a street-based mobile clinic reaches people at elevated risk who might otherwise not pursue treatment.
By targeting individuals with the highest rates of alcohol use and, therefore, the highest risk of severe harm, GRACE has the potential to make a disproportionate impact in improving public health burdens and reducing social and financial costs.
GRACE offers a rapid and appealing benefit to patients by reducing the discomfort of alcohol craving. Craving is a chronic and painful state for people with alcohol dependency. Providing relief centers the patient and their personal agency while also increasing their short-term safety.
Since GLP-1RAs such as semaglutide and tirzepatide are dosed weekly, patients can be informed about the relative ease of compliance.
GLP-1RAs do not require abstinence but dramatically reduce heavy drinking day, even in non-treatment-seeking individuals with AUD. For the vast majority of patients who are hesitant to initiate traditional treatment and are reluctant to commit to abstinence, removing the abstinence requirement can greatly increase the likelihood of adoption.
GLP-1RAs can be self-administered at home with no restrictions and no risk of misuse. A patient can be provided a one-month or longer supply of a GLP-1RA at first visit.
GRACE provides both immediate relief and a foundation of ongoing treatment success. Because GLP-1s reduce cravings across substances, they offer a wide range of benefits to patients who also use other substances in excess.
GRACE can operate within a mobile service model for street-connected individuals. Because GLP-1RAs can be administered anywhere, are dosed only once a week, and have no risk of abuse, they could also be provided through weekly pop-up approaches in high risk areas.
GRACE provides holistic health and personal benefits to patients by improving metabolic health while reducing cravings, anxiety, depression, and neuroinflammation.
Unlike naltrexone, GLP-1RAs do not precipitate opioid withdrawal, so they do not require patients with alcohol poisoning to be screened for OUD. The necessity of screening makes providers less likely to initiate naltrexone for AUD in the ED or EMS settings.
GRACE has a low staffing and financial burden for providing organizations, such as hospitals. GLP-1RAs do not require patients to stay in a facility for any amount of time to detox and, therefore, have a low burden of initiation management from staff. This can dramatically increase the number of locations where effective AUD treatment becomes available.
In sum, GRACE provides a clear short-term benefit to patients, centers their agency, does not require abstinence, and puts them in control of their medication dosing. It may also reduce the cost and staffing burden of treatment provision and prevent future costly emergency encounters.
Potential Challenges of GRACE
Nausea: One common side effect of initiation on a GLP-1RA is nausea and stomach tightness. Nausea is also a side effect of alcohol withdrawal. Providing take-home anti-nausea medicine such as ondansetron or other withdrawal management medication may reduce symptoms. Ondansetron is already commonly used for GLP-1RA initiation. Side effects typically subside after initial dose escalation.
Weight loss concerns in normal weight individuals: GLP-1RAs have been studied for SUD, Parkinson’s disease, diabetes, and elsewhere in normal weight individuals. Unwanted weight loss has not been reported as a barrier to treatment so far. Normal weight patients seem to either maintain weight or are able to titrate to an effective dose that doesn’t cause unwanted weight loss. Weight should be tracked in any clinical trials of GRACE to look for potential relevance to adherence or efficacy. Underweight or malnourished individuals should receive additional monitoring, as there could be further weight loss from lack of appetite or weight gain as opioid use decreases and habits normalize.
Cost: GLP-1RAs are covered for diabetes by most payers and for obesity by some payers, with other related indications like sleep apnea coming soon. GLP-1s are being prescribed off-label for addiction. No payers currently cover this use. Roughly 30% of the US population has obesity or diabetes and may be prescribed with payer coverage on this basis, but that still leaves the majority without coverage. If GRACE is deployed only to people with a covered comorbidity it may still make a significant impact at a public health level. Achieving broad coverage, however, is essential for reaching the full potential. Long-term, that means phase 3 trials for OUD and AUD to achieve an FDA indication. In the medium term there may be a possibility to receive special discounts / coupons from Eli Lilly, Novo Nordisk, or compounding services for use in addiction or emergency care settings. Even if patients were given a monthly supply on site and had to return to the site to get more, GRACE would still represent a substantial improvement in ease of access over current treatment options. Also, as additional evidence accumulates, some payers may be willing to pay for GLP-1RAs through GRACE off-label if it is able to substantially reduce the rates of in-patient treatment of addiction. Virtually any intervention that reduces in-patient treatment is cost-saving to the provider. From a societal standpoint, addictions have tremendous downstream costs for health care, public safety, violent crime, disability, and death. Even a high cost medication provides a huge financial benefit to the public if it meaningfully reduces incidence.
Next Steps for GRACE
GRACE becoming widely adopted will require clinical trials and payer coverage of the medication. Here’s how a timeline can play out.
Short-Term: Prescribing for Comorbidities
Many patients have indicated comorbidities that are already covered by payers. For these patients, GLP-1RAs reduce mortality and provide additional health benefits. People who present with both obesity and AUD, for example, could receive GRACE affordably today. Doing so would require the ability to screen patients at the point of contact for GLP-1RA eligibility for diabetes or obesity. This should only be a modest additional burden on ED or clinical staff since GLP-1s are so widely and safely prescribed by physicians in all settings and typically without requiring blood work.
Due to their massive popularity, GLP-1RAs already have a history of safe use in nearly all substance use disorder populations. For example, more people with AUD already take a GLP-1RA incidentally than take a medication indicated for AUD.
Medium-Term: Clinical Trials of GRACE
To reach broad adoption, GRACE requires clinical trials. A clinical trial of GRACE at an addiction clinic or ED could provide initial real-world evidence. The effect size of GLP-1RAs for alcohol has been large enough that even a relatively small trial is likely to show significance. Our organization, CASPR, is interested in partnering with clinics and investigators to run trials of GRACE. We will be approaching NIDA CTN nodes, among others. Please be in touch if you are interested in collaborating on a study like this.
Long-Term: Phase 3 Trial of GLP-1RAs for AUD and other substance use disorders
For full coverage from private and public insurance, GLP-1RAs require Phase 3 trials and an FDA indication. Funding and running these trials may be the highest ROI opportunity in public health today. Unfortunately, pharma companies have not run Phase 3 trials of GLP-1RAs for addiction because of risk aversion and disinterest in the addiction medication market. It may be left to the public sector to advance these efforts.
CASPR is leading a coalition to run Phase 3 trials GLP-1s for SUD. This is essential for achieving an FDA indication and broad, affordable coverage for patients.
We are eager for thoughts and feedback on GRACE from more providers, clinicians, and researchers. Please be in touch: hello@caspr.org