One of the most important GLP-1 for addiction trials has just begun (and needs another site)
Sue Grigson's trial is looking at semaglutide for OUD-- and they urgently need support to add more patients.
Last year, Dr. Sue Grigson at Penn State announced results of an inpatient study of OUD patients given liraglutide. The trial showed three very important things:
A significant reduction in cravings for opioids in humans given a GLP-1. This confirmed multiple animal studies that had showed the same.
A reduction in cravings, even at the lowest dose of liraglutide. This is an intriguing result because it suggests that low doses may have strong effects, which is crucial for tolerability in normal weight individuals with OUD.
For patients on liraglutide, periods of high stress did not correspond to an increase in cravings. Stress is a huge factor in relapse and decoupling stress from craving could greatly improve patient success.
The trial was run at Caron Treatment Center, where our friend Dr. Erin Deneke runs the research program. The results will be published very soon.
…and now Sue is back!
Dr. Grigson’s new trial will look at semaglutide for OUD in outpatient facilities, which is where most treatment occurs. This study will provide real-world evidence on whether GLP-1s can reduce the use of illicit opioids. It’s the kind of research that could translate quickly into a field-wide shift in practice, and they’ve already enrolled their first participants!
BUT, the trial needs another site.
To ensure that the results will reach statistical significance, Sue is looking to add an additional trial location with more patients. This is especially important for this trial, which will be looking specifically at patients who are receiving methadone or buprenorphine as standard-of-care, and therefore already have substantial craving suppression. The study needs about $860k to open a new site. For a study that has the potential to deliver breakthrough, field-changing results, this should be pocket change— but it’s harder to find than you might think.
CASPR will be making a modest contribution to help, but we are also hoping that some of our readers may be in a position to find funding to get the additional study site up and running. Below, Sue has written up the details of the previous study, the new study, and the need for the 4th site. Please contact her if you may be able to help in any way.
MORE FROM SUE:
I. Completed Pilot Study (UG3 DA050325): Residential Setting: Liraglutide vs. Placebo
Methods. In a fully-randomized, double-blind, placebo-controlled study, patients in residential treatment at the Caron Treatment Centers, Wernersville, PA passed an initial prescreening checklist, completed medically assisted withdrawal, were stratified by their elective choice of MOUD (buprenorphine/naloxone) or No MOUD, and then randomized into placebo or liraglutide treatment conditions: No MOUD Placebo (n=4); No-MOUD Liraglutide (n=3); MOUD Placebo (n=6); MOUD Liraglutide (n=7), for a total of 10 Placebo and 10 Liraglutide treated subjects.
Following initial assessments on Day 1, subcutaneous (SC) liraglutide or placebo was administered daily. Doses were increased from 0.6 mg to 1.2 mg to 1.8 mg every 6 days, then discontinued after a final dose on Day 19. Safety measures (body weight, blood glucose, heart rate, blood pressure, respiratory rate, and oxygen saturation) were taken on the first day of each increasing dose, as well as Day 1 and Days 19 and 21. All participants received a follow up phone call 30 days after leaving treatment to assess interim Adverse Events (AE) or Serious Adverse Events (SAE). Craving was measured via Ecological Momentary Assessment (EMA) using a modified version of the Desires for Drug Questionnaire (Love et al., 1998; Franken, I.H. et al., 2002; Cleveland et al., 2021).
Results. Participants were 84% male, 92% Caucasian.
Safety. Daily treatment with liraglutide did not adversely affect body weight, blood glucose, or cardiorespiratory function in this population. The primary AEs reported involved GI distress. There was one SAE in the Placebo condition and one in the Liraglutide condition, with one individual in each group suffering a non-fatal overdose within 30 days of leaving the Caron Treatment Center. One of these participants also had vasovagal syncope.
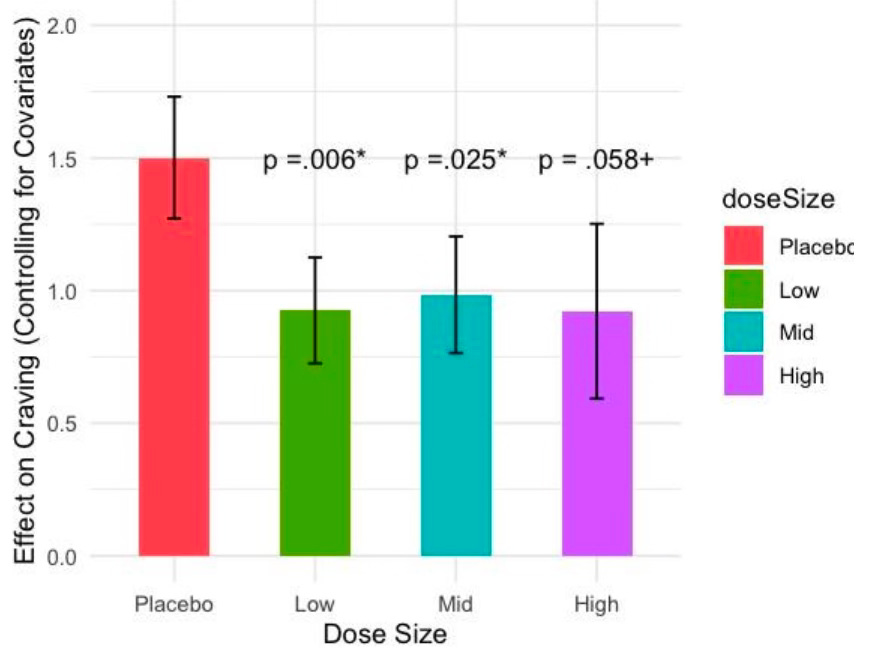
Efficacy. Importantly, while the sample size was small, with the EMA we had 598 data points across 202 person days, affording ample statistical power. Mixed-effects regression grouped by participant showed that liraglutide reduced craving compared to placebo (Cohen’s d = .5, p=0.005), and the drug was effective beginning at the lowest dose administered (Cohen’s d = .5, p=0.005), see Figure 1. Importantly, mixed-effects logistic regression showed that liraglutide reduced opioid craving at times of highest risk, i.e., during the afternoon and evening and, while high stress levels were correlated with high craving for participants on placebo, high stress was not associated with high craving in liraglutide treated participants (p=.031).
II. Clinical Trial Outpatient Setting:
Semaglutide vs. Placebo.
UH3 DA050325 Study Objectives. The purpose of this study is to determine whether 12 weeks of once-weekly treatment with the GLP-1R agonist, semaglutide, will reduce illicit opioid use over a 19-week period (133 days) among individuals in outpatient treatment for OUD, and who are receiving either methadone or buprenorphine/naloxone (Suboxone) maintenance treatment (i.e., medication for opioid use disorder; MOUD). This study will utilize a randomized, double-blind, placebo-controlled, parallel-arm design. Following successful consent and initiation of screening, participants will complete a baseline evaluation and begin a baseline data collection period. If screened into the study, participants will be randomly assigned to semaglutide or placebo control arms in a 1:1 ratio using a permuted-block randomization algorithm stratified by site and MOUD (n=50 methadone + placebo; n=50 methadone + semaglutide; n=50 buprenorphine + placebo; n=50 buprenorphine + semaglutide). After a 1-week baseline period, semaglutide (injector pen) or placebo (empty injector) will be administered SC once per week for 12 weeks, starting at a dose of 0.25 mg SC and advanced on a fixed-flexible dose schedule, based on tolerability, to a target dose of 1.0 mg SC per week, or the maximum tolerated dose if less than 1.0 mg. After the 12 weeks, participants will discontinue semaglutide or placebo and will be observed for a one-week wash-out period, with a follow-up visit 28 days later.
Primary Study Endpoints. The probability of participants being abstinent from illicit and nonprescribed opioids. Each week in the 12-week trial period, participants will be rated as abstinent if both urine test and report by Timeline FollowBack (TLFB) are negative for illicit/non-prescribed opioids, or urine is negative and TLFB missing, or TLFB negative and urine missing; or otherwise not abstinent (either urine positive, or TLFB positive, or both are missing).
Secondary Study Endpoints. The secondary endpoints include: (1) Self-reported opioid craving scores as assessed via smartphone EMA surveys as compared to control group. (2) Self-reported opioid craving as assessed via in-person Cravings Scale scores collected from participants at weekly visits as compared to control group. (3) Binary indicator of sustained abstinence from opioids over the last 4 weeks of the treatment period. (4) Binary indicator of abstinence from stimulants over the last 4 weeks of treatment. (5) Association over the 12-week treatment period between smartphone survey measures of craving and abstinence from opioids and other drugs, as indicated by negative urine drug screens.
III. Proposal. Currently, there are three test sites as listed below, with recruitment beginning January 2025. Here we seek support for the opening of a 4th study site at the Stanley Street Treatment & Resources (SSTAR), Directed by Genie Bailey, MD, Genie@sstar.org, Chief Behavioral Medicine Officer at SSTAR in Fall River, MA 02720. SSTAR has experience with clinical trials and has the patient flow needed (500 patients on methadone and 800 patients on buprenorphine; 30 new buprenorphine patients per month) to allow for recruitment of at least 25 additional participants per year. This would ensure that we meet our recruitment goals at base, but most likely will allow us to exceed those goals and, in so doing, to substantially increase the statistical power of our study and, thereby, the probability of a successful outcome for this important, and first of its kind, outpatient clinical trial designed to test the efficacy of the GLP-1R agonist, semaglutide, for the treatment of OUD in an outpatient setting. If the results show that the GLP-1R agonist significantly increases abstinence from the use of opioids, these data will inform the FDA and potentially contribute to approval of the first new drug for the treatment of OUD in decades, and the first that works independent of the opioid system to reduce craving, relapse, and risk of opioid overdose. The total cost of opening a 4th site is $861,000 in direct costs. We would, of course, be grateful for any contribution toward the opening of the 4th site.
FROM NICHOLAS:
Are you in a position to help this study get additional funding?
It’s hard to overstate the importance of being able to add additional power to this trial through the 4th site. If you are able to contribute to this trial or have a path to additional funding, please contact Sue Grigson.




Thanks, Nicholas!
In a blog post today, Jeff Jackson, the new Attorney General of North Carolina, wrote about a recently-revised settlement agreement in the Purdue Pharma / Sackler family opioids lawsuit, one he believes will be amenable to Federal courts approval:
https://jeffjacksonnc.substack.com/p/the-funding-freeze
"The bottom line? A lot more money for North Carolina. And, by the terms of the agreement, that money will go almost entirely to anti-addiction programs run by individual cities and counties. It’s being channeled directly into the communities that have been hit hardest, with strict rules that it be used to treat addiction or prevent the next generation from falling into this epidemic."
I asked Perplexity.ai, an AI chatbot platform, whether these funds (if approved) could be used to support research activities such as Dr. Grigson's proposed outpatient study. Here's what it came up with when using DeepSeek's R1 model:
https://www.perplexity.ai/search/where-can-one-find-the-propose-c942.cjaT7O6BianiKleOQ
"The proposed $7.4 billion settlement agreement between Purdue Pharma, the Sackler family, and a bipartisan coalition of states is not yet publicly available in full text. However, key details can be pieced together from official announcements and court filings ...
"While the settlement prioritizes opioid treatment, prevention, and recovery programs, its language does not explicitly prohibit or mandate research funding. ...
"Clinical trials like outpatient studies of GLP-1 agonists (e.g., liraglutide) for opioid use disorder could qualify under *treatment innovation* (emphasis added - Aron) if aligned with state-approved abatement strategies. However, this would depend on local guidelines, as the settlement itself does not specify research funding."
Am hoping you might be able to share this with Dr. Grigson and others, in case this might prove promising. Thanks!