Phase 3 trials of supplements and off-patent drugs can generate a windfall for the public
Better medicines, lower costs, and faster time to market.
There’s essentially only one way that medicines become available for your doctor to prescribe and for your insurance to cover: large phase III trials followed by FDA approval.
Nearly every phase III trial is run by a biotech or pharma company, so if industry doesn’t test a medicine, we almost never really know if it works. Supplements and drugs with expired patents don’t get Phase III trials because there’s not enough incentive to run a trial on medicine without patent exclusivity. That means that hundreds of safe and effective treatments are sitting on the shelf, unstudied.
I’ve been frustrated and perplexed about this problem years, and there have been proposals to address it, but I think that through understanding how similar the obstacle is that supplements and off-patent therapeutics face, we can find a solution that is broadly appealing across the political spectrum:
To solve this gap and open up new treatments and dramatic cost savings, we propose the creation of an NIH High-Leverage Trials (HILT) Program for off-patent drug repurposing and supplements. HILT would fund and run definitive large scale trials and, when appropriate, advance regulatory approvals to ensure broad patient access.
The medical opportunity and cost savings potential are tremendous. Here’s my proposal, published with The Good Science Project:
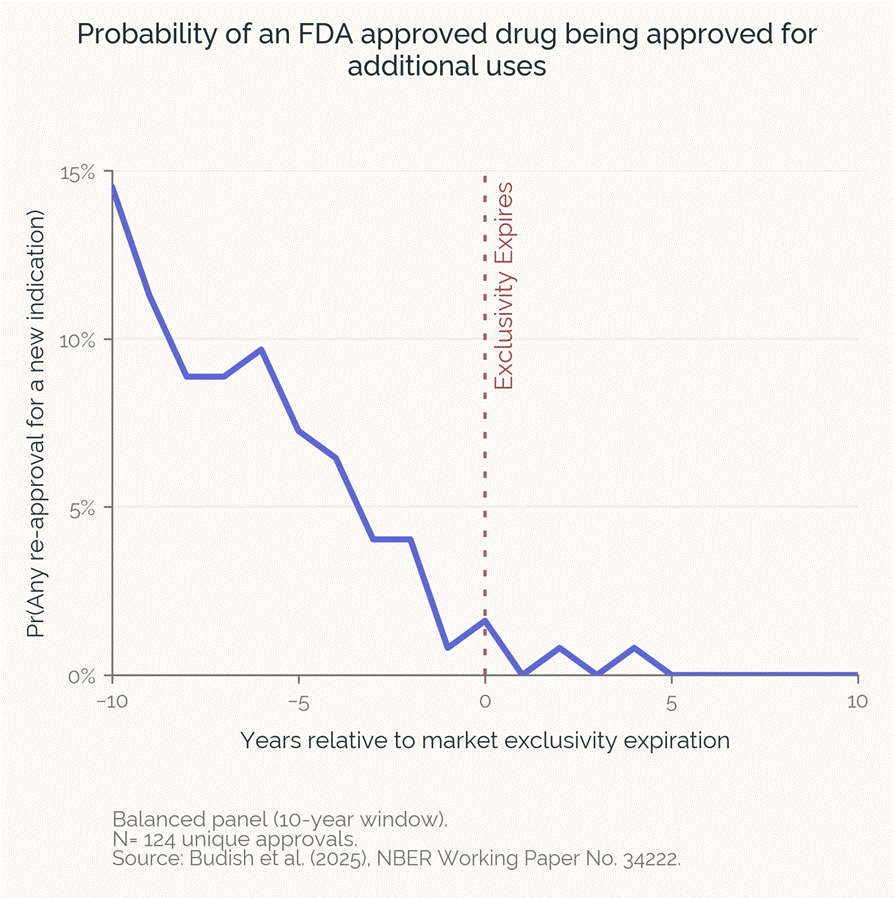
Here’s a chart from the piece, based on data from CASPR advisor Maya Durvasula’s recent paper on new uses for existing drugs.
The full article is here: Proposing an NIH High-Leverage Trials (HILT) Program: Large-scale Research for Repurposing and Supplements.
Thank you to Stuart Buck of The Good Science Project for invaluable feedback and for publishing my paper today on his substack, which I highly recommend.



