“There were some dark days”: Why there have been shortages of naltrexone, a key medication for alcohol use disorder
Our reporting reveals we should blame increased demand, brittle supply chains, and the DEA.
Last November, Jonathan Hunt-Glassman noticed something unusual. The founder of OAR Health, a company that helps people with alcohol use disorder get and fill naltrexone prescriptions, started hearing that there was a shortage of the 40-year old FDA-approved medicine. “We work with online pharmacies to fill prescriptions for our patients and out of the blue, they started saying we might have to stop filling orders,” he told Recursive Adaptation.
Ultimately, OAR was able to fill every prescription, but it required sacrifice. The company stopped marketing and stopped accepting new patients for periods of time, while some existing patients took a 30-day supply instead of the usual 90-day. Other patients who were using naltrexone on a spot basis rather than daily held off on their shipments entirely. “There were some dark days between November and February,” Hunt-Glassman said.
His experience at OAR wasn’t unique. Across the country, there was a largely unexplained naltrexone tablet shortage starting in late 2023, leaving patients scrambling. Social media is full of posts detailing individual struggles to procure the drug.
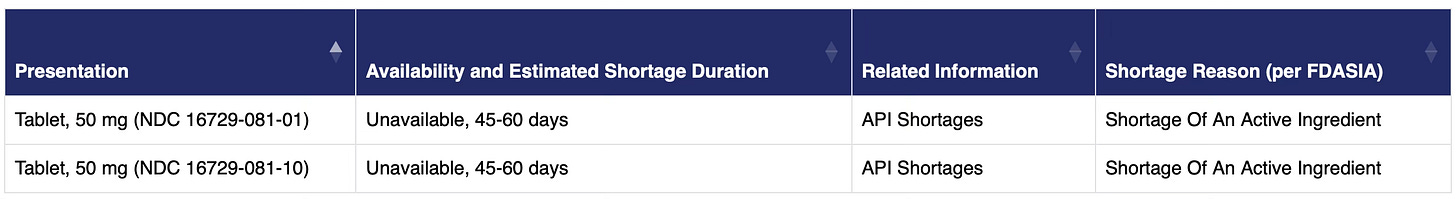
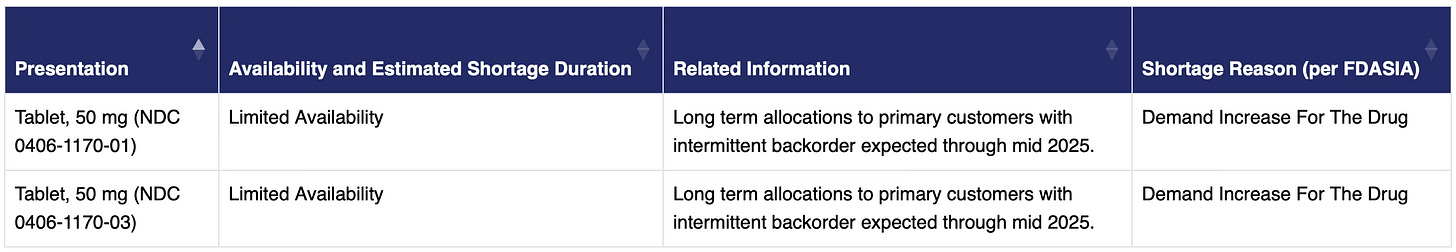
While supply has improved, it’s still not where it needs to be. The DEA wouldn’t comment on the reasons for the shortage, simply sending a link to an FDA Drug Shortage list where three of the five companies selling naltrexone reported that 50 mb tablets were “Unavailable” or in “Limited Availability.” The reasons given include “Demand Increase For The Drug” and “Shortage Of An Active Ingredient.”
Demand for naltrexone has increased, which is part of the story. Jeff Harris is a vice president and general manager at drug manufacturer Alkermes. The company makes Vivitrol, a monthly shot of extended-release naltrexone. “During COVID, a lot of people observed the increased rate of drinking and alcohol-related problems,” he told us. “I think it was predictable that once the early phase of COVID passed by, there would start to be more demand for treatment.” While Alkermes didn’t run into any shortages because, Harris says, of the three year-plus planning cycles, they did see some providers switch patients to Vivitrol due to a lack of oral naltrexone pills.
And then there’s the “Shortage Of An Active Ingredient” part of the equation. The main precursor, or API, to naltrexone hydrochloride is thebaine, described as “a morphinane alkaloid and an organic heteropentacyclic compound.” Two notes here: There are only a few countries from which thebaine can legally be imported, and thebaine is used to make other popular drugs such as hydrocodone, oxycodone, oxymorphone, naloxone, and buprenorphine in addition to naltrexone. The result is that even though naltrexone does not have addictive qualities, the API can be used to make other drugs that do, and, therefore, the amount that is imported is regulated by DEA.
Furthermore, naltrexone is not a high revenue medication, costing just $44 for a 30-day supply. It was also only the 254th most-prescribed drug in the United States in 2021 and not in the top 300 in 2022. And Naltrexone is difficult to manufacture, taking six months or more to make new pills. Even perfect supply chains will take time to ramp up to the new higher demand.
“My own guess is that naltrexone is way down the totem pole on the list of priorities.”
It’s easy to see how a brittle supply chain, an increase of demand for precursor, and a low priority adds up to a naltrexone shortage. “My own guess is that naltrexone is way down the totem pole on the list of priorities,” Percy Menzies, an executive who helped market naltrexone at Dupont and current president and founder of Assisted Recovery Centers of America, said during a phone interview “Whatever limited quantity of thebaine a company is going to make other medications that have a much higher profit margin.”
But for people who take it every day, naltrexone is vital. Shortages shouldn’t exist. Given the importance of consistent access to patients using oral naltrexone and the fact that naltrexone is non-addictive, it would be smart to find a way to ensure ongoing manufacturer access to the API. Some lawmakers are pushing to remove burdensome monitoring of buprenorphine that limits access. Perhaps these same representatives could look to a similar bill removing import limits on the API when intended for naltrexone production.
Recent posts you may have missed: